

Hybridization is vital to understand the molecular geometry of the compound. Each Chlorine atom has six valence electrons after the bonds are formed. Four lines in the structure represent four bonds while dots around the Chlorine atom represent valence electrons. So there are a total of 24 non-bonding or 12 lone pairs of electrons in CCl4. Total 8 electrons make the bonds while others are non-bonding pairs of electrons.

Similarly, a single electron form each Chlorine atom participate in bond formation. As there are four molecules of Chlorine, we will calculate the number of valence electrons accordingly.Īll four valence electrons of Carbon participate in the bond formation. Lewis theory is based on the octet rule, which states that an atom should have eight electrons in its outer shell to be stable.įor the Lewis structure of CCl4 first, let’s calculate the total valence electrons.Ĭarbon has four valence electrons and each Chlorine atom has seven valence electrons. While the dots represent the non-bonding electrons. Sticks or straight lines represent the bonds. The Lewis structure is the pictorial representation of the valence electrons that participate in the bond formation as well as the ones that don’t. The bonding, as well as non-bonding electrons collectively, are called valence electrons. The ones that do not participate in it are known by the term non-bonding or lone pair of electrons. The electrons that participate in forming the bonds are known as the bonding pair of electrons. G.N Lewis first proposed this theory in 1916 that helps in understanding the involvement of electrons informing the structure of the chemical.
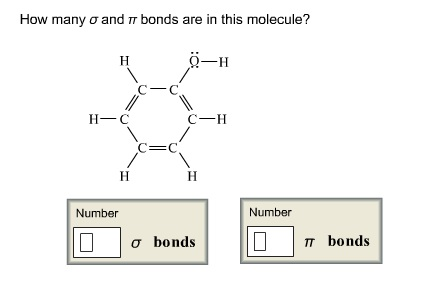
In chemistry, the basis of understanding any property of the compound depends on its lewis structure.


 0 kommentar(er)
0 kommentar(er)
